Sharp EL-546L: Difference between revisions
Jump to navigation
Jump to search
No edit summary |
|||
| (15 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
I have a Sharp EL-546L scientific calculator. The images on the right are of its Quick Reference Card. [[#Physical Constants|Physical Constants]] and [[#Metric Conversions|Metric Conversions]] are detailed below. | I have a Sharp EL-546L scientific calculator in my [[lab]] with my [[computers]]. | ||
= Affiliate links = | |||
If you want to buy, see here: | |||
{|class=wikitable | |||
! Vendor !! Product | |||
|- | |||
| eBay USA || [https://ebay.us/46vqZ2 Sharp EL-546L] | |||
|} | |||
= Documentation = | |||
I keep my documentation for this equipment here: | |||
* /home/jj5/electronics/equipment/calculator-Sharp-EL-546L/ | |||
= Details = | |||
The images on the right are of its Quick Reference Card. [[#Physical Constants|Physical Constants]] and [[#Metric Conversions|Metric Conversions]] are detailed below. | |||
<span style="color:black;">[[File:EL-546L-quick-reference-card.jpg|frame|Substitute Algebra and Playback & Editing]]</span> | <span style="color:black;">[[File:EL-546L-quick-reference-card.jpg|frame|Substitute Algebra and Playback & Editing]]</span> | ||
| Line 7: | Line 27: | ||
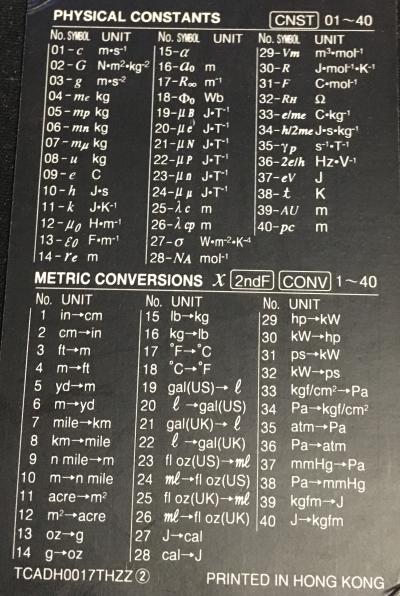
== Physical Constants == | == Physical Constants == | ||
Note: it seems only the absolute values are available from the calculator, even for constants which should generally be negative; so keep | Note: it seems only the absolute values are available from the calculator, even for constants which should generally be negative; so keep an eye on that. The values shown are the values the calculator provides, other sources may vary. | ||
{|class="wikitable" | {|class="wikitable sortable" | ||
! No. | ! No. | ||
! Symbol | ! Symbol | ||
! Unit | ! Unit | ||
! Value | ! Value | ||
! Magnitude | |||
! Name | ! Name | ||
|- | |- | ||
| Line 20: | Line 41: | ||
|class="math"| <math>m \cdot s^{-1}</math> | |class="math"| <math>m \cdot s^{-1}</math> | ||
|class="right"| 2.99792458 x 10<sup>8</sup> | |class="right"| 2.99792458 x 10<sup>8</sup> | ||
|class="right"| 8 | |||
| [https://en.wikipedia.org/wiki/Speed_of_light speed of light] | | [https://en.wikipedia.org/wiki/Speed_of_light speed of light] | ||
|- | |- | ||
| Line 26: | Line 48: | ||
|class="math"| <math>N \cdot m^2 \cdot kg^{-2}</math> | |class="math"| <math>N \cdot m^2 \cdot kg^{-2}</math> | ||
|class="right"| 6.67259 x 10<sup>-11</sup> | |class="right"| 6.67259 x 10<sup>-11</sup> | ||
|class="right"| -11 | |||
| [https://en.wikipedia.org/wiki/Gravitational_constant gravitational constant] | | [https://en.wikipedia.org/wiki/Gravitational_constant gravitational constant] | ||
|- | |- | ||
| Line 32: | Line 55: | ||
|class="math"| <math>m \cdot s^{-2}</math> | |class="math"| <math>m \cdot s^{-2}</math> | ||
|class="right"| 9.80665 | |class="right"| 9.80665 | ||
|class="right"| 0 | |||
| [https://en.wikipedia.org/wiki/Gravity_of_Earth gravity of Earth] | | [https://en.wikipedia.org/wiki/Gravity_of_Earth gravity of Earth] | ||
|- | |- | ||
| Line 38: | Line 62: | ||
|class="math"| <math>kg</math> | |class="math"| <math>kg</math> | ||
|class="right"| 9.1093897 x 10<sup>-31</sup> | |class="right"| 9.1093897 x 10<sup>-31</sup> | ||
|class="right"| -31 | |||
| [https://en.wikipedia.org/wiki/Electron mass of an electron] | | [https://en.wikipedia.org/wiki/Electron mass of an electron] | ||
|- | |- | ||
| Line 44: | Line 69: | ||
|class="math"| <math>kg</math> | |class="math"| <math>kg</math> | ||
|class="right"| 1.6726231 x 10<sup>-27</sup> | |class="right"| 1.6726231 x 10<sup>-27</sup> | ||
|class="right"| -27 | |||
| [https://en.wikipedia.org/wiki/Proton mass of a proton] | | [https://en.wikipedia.org/wiki/Proton mass of a proton] | ||
|- | |- | ||
| Line 50: | Line 76: | ||
|class="math"| <math>kg</math> | |class="math"| <math>kg</math> | ||
|class="right"| 1.6749286 x 10<sup>-27</sup> | |class="right"| 1.6749286 x 10<sup>-27</sup> | ||
|class="right"| -27 | |||
| [https://en.wikipedia.org/wiki/Neutron mass of a neutron] | | [https://en.wikipedia.org/wiki/Neutron mass of a neutron] | ||
|- | |- | ||
| Line 56: | Line 83: | ||
|class="math"| <math>kg</math> | |class="math"| <math>kg</math> | ||
|class="right"| 1.8835327 x 10<sup>-28</sup> | |class="right"| 1.8835327 x 10<sup>-28</sup> | ||
|class="right"| -28 | |||
| [https://en.wikipedia.org/wiki/Muon mass of a muon] | | [https://en.wikipedia.org/wiki/Muon mass of a muon] | ||
|- | |- | ||
| Line 62: | Line 90: | ||
|class="math"| <math>kg</math> | |class="math"| <math>kg</math> | ||
|class="right"| 1.6605402 x 10<sup>-27</sup> | |class="right"| 1.6605402 x 10<sup>-27</sup> | ||
|class="right"| -27 | |||
| [https://en.wikipedia.org/wiki/Atomic_mass_unit atomic mass unit] | | [https://en.wikipedia.org/wiki/Atomic_mass_unit atomic mass unit] | ||
|- | |- | ||
| Line 68: | Line 97: | ||
|class="math"| <math>C</math> | |class="math"| <math>C</math> | ||
|class="right"| 1.60217733 x 10<sup>-19</sup> | |class="right"| 1.60217733 x 10<sup>-19</sup> | ||
|class="right"| -19 | |||
| [https://en.wikipedia.org/wiki/Electron charge of an electron] | | [https://en.wikipedia.org/wiki/Electron charge of an electron] | ||
|- | |- | ||
| Line 74: | Line 104: | ||
|class="math"| <math>J \cdot s</math> | |class="math"| <math>J \cdot s</math> | ||
|class="right"| 6.6260755 x 10<sup>-34</sup> | |class="right"| 6.6260755 x 10<sup>-34</sup> | ||
|class="right"| -34 | |||
| [https://en.wikipedia.org/wiki/Planck_constant Planck constant] | | [https://en.wikipedia.org/wiki/Planck_constant Planck constant] | ||
|- | |- | ||
| Line 80: | Line 111: | ||
|class="math"| <math>J \cdot K^{-1}</math> | |class="math"| <math>J \cdot K^{-1}</math> | ||
|class="right"| 1.380658 x 10<sup>-23</sup> | |class="right"| 1.380658 x 10<sup>-23</sup> | ||
|class="right"| -23 | |||
| [https://en.wikipedia.org/wiki/Boltzmann_constant Boltzmann constant] | | [https://en.wikipedia.org/wiki/Boltzmann_constant Boltzmann constant] | ||
|- | |- | ||
| Line 86: | Line 118: | ||
|class="math"| <math>H \cdot m^{-1}</math> | |class="math"| <math>H \cdot m^{-1}</math> | ||
|class="right"| 1.256637061 x 10<sup>-6</sup> | |class="right"| 1.256637061 x 10<sup>-6</sup> | ||
|class="right"| -6 | |||
| [https://en.wikipedia.org/wiki/Vacuum_permeability vacuum permeability] | | [https://en.wikipedia.org/wiki/Vacuum_permeability vacuum permeability] | ||
|- | |- | ||
| Line 92: | Line 125: | ||
|class="math"| <math>F \cdot m^{-1}</math> | |class="math"| <math>F \cdot m^{-1}</math> | ||
|class="right"| 8.854187817 x 10<sup>-12</sup> | |class="right"| 8.854187817 x 10<sup>-12</sup> | ||
|class="right"| -12 | |||
| [https://en.wikipedia.org/wiki/Vacuum_permittivity vacuum permittivity] | | [https://en.wikipedia.org/wiki/Vacuum_permittivity vacuum permittivity] | ||
|- | |- | ||
| Line 98: | Line 132: | ||
|class="math"| <math>m</math> | |class="math"| <math>m</math> | ||
|class="right"| 2.81794092 x 10<sup>-15</sup> | |class="right"| 2.81794092 x 10<sup>-15</sup> | ||
|class="right"| -15 | |||
| [https://en.wikipedia.org/wiki/Classical_electron_radius classical electron radius] | | [https://en.wikipedia.org/wiki/Classical_electron_radius classical electron radius] | ||
|- | |- | ||
| Line 104: | Line 139: | ||
|class="math"| | |class="math"| | ||
|class="right"| 7.29735308 x 10<sup>-3</sup> | |class="right"| 7.29735308 x 10<sup>-3</sup> | ||
|class="right"| -3 | |||
| [https://en.wikipedia.org/wiki/Fine-structure_constant fine-structure constant] | | [https://en.wikipedia.org/wiki/Fine-structure_constant fine-structure constant] | ||
|- | |- | ||
| Line 110: | Line 146: | ||
|class="math"| <math>m</math> | |class="math"| <math>m</math> | ||
|class="right"| 5.29177249 x 10<sup>-11</sup> | |class="right"| 5.29177249 x 10<sup>-11</sup> | ||
|class="right"| -11 | |||
| [https://en.wikipedia.org/wiki/Bohr_radius Bohr radius] | | [https://en.wikipedia.org/wiki/Bohr_radius Bohr radius] | ||
|- | |- | ||
| Line 116: | Line 153: | ||
|class="math"| <math>m^{-1}</math> | |class="math"| <math>m^{-1}</math> | ||
|class="right"| 1.097373153 x 10<sup>7</sup> | |class="right"| 1.097373153 x 10<sup>7</sup> | ||
|class="right"| 7 | |||
| [https://en.wikipedia.org/wiki/Rydberg_constant Rydberg constant] | | [https://en.wikipedia.org/wiki/Rydberg_constant Rydberg constant] | ||
|- | |- | ||
| Line 122: | Line 160: | ||
|class="math"| <math>Wb</math> | |class="math"| <math>Wb</math> | ||
|class="right"| 2.06783461 x 10<sup>-15</sup> | |class="right"| 2.06783461 x 10<sup>-15</sup> | ||
|class="right"| -15 | |||
| [https://en.wikipedia.org/wiki/Magnetic_flux_quantum magnetic flux quantum] | | [https://en.wikipedia.org/wiki/Magnetic_flux_quantum magnetic flux quantum] | ||
|- | |- | ||
| Line 128: | Line 167: | ||
|class="math"| <math>J \cdot T^{-1}</math> | |class="math"| <math>J \cdot T^{-1}</math> | ||
|class="right"| 9.2740154 x 10<sup>-24</sup> | |class="right"| 9.2740154 x 10<sup>-24</sup> | ||
|class="right"| -24 | |||
| [https://en.wikipedia.org/wiki/Bohr_magneton Bohr magneton] | | [https://en.wikipedia.org/wiki/Bohr_magneton Bohr magneton] | ||
|- | |- | ||
| Line 134: | Line 174: | ||
|class="math"| <math>J \cdot T^{-1}</math> | |class="math"| <math>J \cdot T^{-1}</math> | ||
|class="right"| 9.2847701 x 10<sup>-24</sup> | |class="right"| 9.2847701 x 10<sup>-24</sup> | ||
|class="right"| -24 | |||
| [https://en.wikipedia.org/wiki/Electron_magnetic_moment electron magnetic moment] | | [https://en.wikipedia.org/wiki/Electron_magnetic_moment electron magnetic moment] | ||
|- | |- | ||
| Line 140: | Line 181: | ||
|class="math"| <math>J \cdot T^{-1}</math> | |class="math"| <math>J \cdot T^{-1}</math> | ||
|class="right"| 5.0507866 x 10<sup>-27</sup> | |class="right"| 5.0507866 x 10<sup>-27</sup> | ||
|class="right"| -27 | |||
| [https://en.wikipedia.org/wiki/Nuclear_magneton nuclear magneton] | | [https://en.wikipedia.org/wiki/Nuclear_magneton nuclear magneton] | ||
|- | |- | ||
| Line 146: | Line 188: | ||
|class="math"| <math>J \cdot T^{-1}</math> | |class="math"| <math>J \cdot T^{-1}</math> | ||
|class="right"| 1.41060761 x 10<sup>-26</sup> | |class="right"| 1.41060761 x 10<sup>-26</sup> | ||
|class="right"| -26 | |||
| [https://en.wikipedia.org/wiki/Proton_magnetic_moment proton magnetic moment] | | [https://en.wikipedia.org/wiki/Proton_magnetic_moment proton magnetic moment] | ||
|- | |- | ||
| Line 152: | Line 195: | ||
|class="math"| <math>J \cdot T^{-1}</math> | |class="math"| <math>J \cdot T^{-1}</math> | ||
|class="right"| 9.6623707 x 10<sup>-27</sup> | |class="right"| 9.6623707 x 10<sup>-27</sup> | ||
|class="right"| -27 | |||
| [https://en.wikipedia.org/wiki/Neutron_magnetic_moment neutron magnetic moment] | | [https://en.wikipedia.org/wiki/Neutron_magnetic_moment neutron magnetic moment] | ||
|- | |- | ||
| Line 158: | Line 202: | ||
|class="math"| <math>J \cdot T^{-1}</math> | |class="math"| <math>J \cdot T^{-1}</math> | ||
|class="right"| 4.4904514 x 10<sup>-26</sup> | |class="right"| 4.4904514 x 10<sup>-26</sup> | ||
|class="right"| -26 | |||
| [https://en.wikipedia.org/wiki/Anomalous_magnetic_dipole_moment#Muon muon magnetic moment] | | [https://en.wikipedia.org/wiki/Anomalous_magnetic_dipole_moment#Muon muon magnetic moment] | ||
|- | |- | ||
| Line 164: | Line 209: | ||
|class="math"| <math>m</math> | |class="math"| <math>m</math> | ||
|class="right"| 2.42631058 x 10<sup>-12</sup> | |class="right"| 2.42631058 x 10<sup>-12</sup> | ||
|class="right"| -12 | |||
| [https://en.wikipedia.org/wiki/Compton_wavelength Compton wavelength] | | [https://en.wikipedia.org/wiki/Compton_wavelength Compton wavelength] | ||
|- | |- | ||
| Line 170: | Line 216: | ||
|class="math"| <math>m</math> | |class="math"| <math>m</math> | ||
|class="right"| 1.32141002 x 10<sup>-15</sup> | |class="right"| 1.32141002 x 10<sup>-15</sup> | ||
|class="right"| -15 | |||
| [https://en.wikipedia.org/wiki/Compton_wavelength proton Compton wavelength] | | [https://en.wikipedia.org/wiki/Compton_wavelength proton Compton wavelength] | ||
|- | |- | ||
| Line 176: | Line 223: | ||
|class="math"| <math>W \cdot m^{-2} \cdot K^{-4}</math> | |class="math"| <math>W \cdot m^{-2} \cdot K^{-4}</math> | ||
|class="right"| 5.67051 x 10<sup>-8</sup> | |class="right"| 5.67051 x 10<sup>-8</sup> | ||
|class="right"| -8 | |||
| [https://en.wikipedia.org/wiki/Stefan%E2%80%93Boltzmann_constant Stefan–Boltzmann constant] | | [https://en.wikipedia.org/wiki/Stefan%E2%80%93Boltzmann_constant Stefan–Boltzmann constant] | ||
|- | |- | ||
| Line 182: | Line 230: | ||
|class="math"| <math>mol^{-1}</math> | |class="math"| <math>mol^{-1}</math> | ||
|class="right"| 6.0221367 x 10<sup>23</sup> | |class="right"| 6.0221367 x 10<sup>23</sup> | ||
|class="right"| 23 | |||
| [https://en.wikipedia.org/wiki/Avogadro_constant Avogadro constant] | | [https://en.wikipedia.org/wiki/Avogadro_constant Avogadro constant] | ||
|- | |- | ||
| Line 188: | Line 237: | ||
|class="math"| <math>m^3 \cdot mol^{-1}</math> | |class="math"| <math>m^3 \cdot mol^{-1}</math> | ||
|class="right"| 2.24141 x 10<sup>-2</sup> | |class="right"| 2.24141 x 10<sup>-2</sup> | ||
|class="right"| -2 | |||
| [https://en.wikipedia.org/wiki/Molar_volume molar volume] | | [https://en.wikipedia.org/wiki/Molar_volume molar volume] | ||
|- | |- | ||
| Line 194: | Line 244: | ||
|class="math"| <math>J \cdot mol^{-1} \cdot K^{-1}</math> | |class="math"| <math>J \cdot mol^{-1} \cdot K^{-1}</math> | ||
|class="right"| 8.31451 | |class="right"| 8.31451 | ||
|class="right"| 0 | |||
| [https://en.wikipedia.org/wiki/Gas_constant gas constant] | | [https://en.wikipedia.org/wiki/Gas_constant gas constant] | ||
|- | |- | ||
| Line 200: | Line 251: | ||
|class="math"| <math>C \cdot mol^{-1}</math> | |class="math"| <math>C \cdot mol^{-1}</math> | ||
|class="right"| 9.6485309 x 10<sup>4</sup> | |class="right"| 9.6485309 x 10<sup>4</sup> | ||
|class="right"| 4 | |||
| [https://en.wikipedia.org/wiki/Faraday_constant Faraday constant] | | [https://en.wikipedia.org/wiki/Faraday_constant Faraday constant] | ||
|- | |- | ||
| Line 206: | Line 258: | ||
|class="math"| <math>\Omega</math> | |class="math"| <math>\Omega</math> | ||
|class="right"| 2.58128056 x 10<sup>4</sup> | |class="right"| 2.58128056 x 10<sup>4</sup> | ||
|class="right"| 4 | |||
| [https://en.wikipedia.org/wiki/Quantum_Hall_effect quantized Hall resistance] | | [https://en.wikipedia.org/wiki/Quantum_Hall_effect quantized Hall resistance] | ||
|- | |- | ||
| Line 212: | Line 265: | ||
|class="math"| <math>C \cdot kg^{-1}</math> | |class="math"| <math>C \cdot kg^{-1}</math> | ||
|class="right"| 1.75881962 x 10<sup>11</sup> | |class="right"| 1.75881962 x 10<sup>11</sup> | ||
|class="right"| 11 | |||
| [https://duckduckgo.com/?q=electron+specific+charge electron specific charge] | | [https://duckduckgo.com/?q=electron+specific+charge electron specific charge] | ||
|- | |- | ||
| Line 218: | Line 272: | ||
|class="math"| <math>J \cdot s \cdot kg^{-1}</math> | |class="math"| <math>J \cdot s \cdot kg^{-1}</math> | ||
|class="right"| 3.63694807 x 10<sup>-4</sup> | |class="right"| 3.63694807 x 10<sup>-4</sup> | ||
|class="right"| -4 | |||
| [https://duckduckgo.com/?q=quantum+of+circulation quantum of circulation] | | [https://duckduckgo.com/?q=quantum+of+circulation quantum of circulation] | ||
|- | |- | ||
| Line 224: | Line 279: | ||
|class="math"| <math>s^{-1} \cdot T^{-1}</math> | |class="math"| <math>s^{-1} \cdot T^{-1}</math> | ||
|class="right"| 2.67522128 x 10<sup>8</sup> | |class="right"| 2.67522128 x 10<sup>8</sup> | ||
|class="right"| 8 | |||
| [https://duckduckgo.com/?q=gyromagnetic+ratio+of+proton gyromagnetic ratio of proton] | | [https://duckduckgo.com/?q=gyromagnetic+ratio+of+proton gyromagnetic ratio of proton] | ||
|- | |- | ||
| Line 230: | Line 286: | ||
|class="math"| <math>Hz \cdot V^{-1}</math> | |class="math"| <math>Hz \cdot V^{-1}</math> | ||
|class="right"| 4.8359767 x 10<sup>14</sup> | |class="right"| 4.8359767 x 10<sup>14</sup> | ||
|class="right"| 14 | |||
| [https://duckduckgo.com/?q=Josephson+frequency-voltage+quotient Josephson frequency-voltage quotient] | | [https://duckduckgo.com/?q=Josephson+frequency-voltage+quotient Josephson frequency-voltage quotient] | ||
|- | |- | ||
| Line 236: | Line 293: | ||
|class="math"| <math>J</math> | |class="math"| <math>J</math> | ||
|class="right"| 1.60217733 x 10<sup>-19</sup> | |class="right"| 1.60217733 x 10<sup>-19</sup> | ||
|class="right"| -19 | |||
| [https://en.wikipedia.org/wiki/Elementary_charge elementary charge] | | [https://en.wikipedia.org/wiki/Elementary_charge elementary charge] | ||
|- | |- | ||
|class="right"| 38 | |class="right"| 38 | ||
|class="math"| <math>t</math> | |class="math"| <math>t</math> | ||
|class="math"| <math> | |class="math"| <math>^{\circ}C</math> | ||
|class="right"| 2.7315 x 10<sup>2</sup> | |class="right"| 2.7315 x 10<sup>2</sup> | ||
|class="right"| 2 | |||
| [https://en.wikipedia.org/wiki/Absolute_zero absolute zero] | | [https://en.wikipedia.org/wiki/Absolute_zero absolute zero] | ||
|- | |- | ||
| Line 248: | Line 307: | ||
|class="math"| <math>m</math> | |class="math"| <math>m</math> | ||
|class="right"| 1.4959787 x 10<sup>11</sup> | |class="right"| 1.4959787 x 10<sup>11</sup> | ||
|class="right"| 11 | |||
| [https://en.wikipedia.org/wiki/Astronomical_unit astronomical unit] | | [https://en.wikipedia.org/wiki/Astronomical_unit astronomical unit] | ||
|- | |- | ||
| Line 254: | Line 314: | ||
|class="math"| <math>m</math> | |class="math"| <math>m</math> | ||
|class="right"| 3.0856776 x 10<sup>16</sup> | |class="right"| 3.0856776 x 10<sup>16</sup> | ||
|class="right"| 16 | |||
| [https://en.wikipedia.org/wiki/Parsec parsec] | | [https://en.wikipedia.org/wiki/Parsec parsec] | ||
|} | |} | ||
Latest revision as of 10:09, 31 October 2023
I have a Sharp EL-546L scientific calculator in my lab with my computers.
Affiliate links
If you want to buy, see here:
| Vendor | Product |
|---|---|
| eBay USA | Sharp EL-546L |
Documentation
I keep my documentation for this equipment here:
- /home/jj5/electronics/equipment/calculator-Sharp-EL-546L/
Details
The images on the right are of its Quick Reference Card. Physical Constants and Metric Conversions are detailed below.
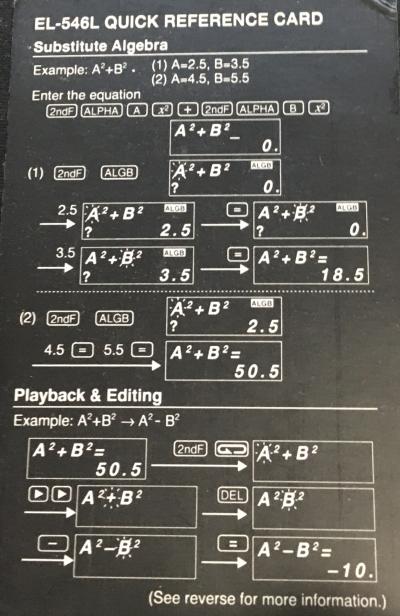
Physical Constants
Note: it seems only the absolute values are available from the calculator, even for constants which should generally be negative; so keep an eye on that. The values shown are the values the calculator provides, other sources may vary.
| No. | Symbol | Unit | Value | Magnitude | Name |
|---|---|---|---|---|---|
| 01 | 2.99792458 x 108 | 8 | speed of light | ||
| 02 | 6.67259 x 10-11 | -11 | gravitational constant | ||
| 03 | 9.80665 | 0 | gravity of Earth | ||
| 04 | 9.1093897 x 10-31 | -31 | mass of an electron | ||
| 05 | 1.6726231 x 10-27 | -27 | mass of a proton | ||
| 06 | 1.6749286 x 10-27 | -27 | mass of a neutron | ||
| 07 | 1.8835327 x 10-28 | -28 | mass of a muon | ||
| 08 | 1.6605402 x 10-27 | -27 | atomic mass unit | ||
| 09 | 1.60217733 x 10-19 | -19 | charge of an electron | ||
| 10 | 6.6260755 x 10-34 | -34 | Planck constant | ||
| 11 | 1.380658 x 10-23 | -23 | Boltzmann constant | ||
| 12 | 1.256637061 x 10-6 | -6 | vacuum permeability | ||
| 13 | 8.854187817 x 10-12 | -12 | vacuum permittivity | ||
| 14 | 2.81794092 x 10-15 | -15 | classical electron radius | ||
| 15 | 7.29735308 x 10-3 | -3 | fine-structure constant | ||
| 16 | 5.29177249 x 10-11 | -11 | Bohr radius | ||
| 17 | 1.097373153 x 107 | 7 | Rydberg constant | ||
| 18 | 2.06783461 x 10-15 | -15 | magnetic flux quantum | ||
| 19 | 9.2740154 x 10-24 | -24 | Bohr magneton | ||
| 20 | 9.2847701 x 10-24 | -24 | electron magnetic moment | ||
| 21 | 5.0507866 x 10-27 | -27 | nuclear magneton | ||
| 22 | 1.41060761 x 10-26 | -26 | proton magnetic moment | ||
| 23 | 9.6623707 x 10-27 | -27 | neutron magnetic moment | ||
| 24 | 4.4904514 x 10-26 | -26 | muon magnetic moment | ||
| 25 | 2.42631058 x 10-12 | -12 | Compton wavelength | ||
| 26 | 1.32141002 x 10-15 | -15 | proton Compton wavelength | ||
| 27 | 5.67051 x 10-8 | -8 | Stefan–Boltzmann constant | ||
| 28 | 6.0221367 x 1023 | 23 | Avogadro constant | ||
| 29 | 2.24141 x 10-2 | -2 | molar volume | ||
| 30 | 8.31451 | 0 | gas constant | ||
| 31 | 9.6485309 x 104 | 4 | Faraday constant | ||
| 32 | 2.58128056 x 104 | 4 | quantized Hall resistance | ||
| 33 | 1.75881962 x 1011 | 11 | electron specific charge | ||
| 34 | 3.63694807 x 10-4 | -4 | quantum of circulation | ||
| 35 | 2.67522128 x 108 | 8 | gyromagnetic ratio of proton | ||
| 36 | 4.8359767 x 1014 | 14 | Josephson frequency-voltage quotient | ||
| 37 | 1.60217733 x 10-19 | -19 | elementary charge | ||
| 38 | 2.7315 x 102 | 2 | absolute zero | ||
| 39 | 1.4959787 x 1011 | 11 | astronomical unit | ||
| 40 | 3.0856776 x 1016 | 16 | parsec |
Metric Conversions
| No. | From Unit | To Unit |
|---|---|---|
| 1 | in | cm |
| 2 | cm | in |
| 3 | ft | m |
| 4 | m | ft |
| 5 | yd | m |
| 6 | m | yd |
| 7 | mile | km |
| 8 | km | mile |
| 9 | n mile | m |
| 10 | m | n mile |
| 11 | acre | m2 |
| 12 | m2 | acre |
| 13 | oz | g |
| 14 | g | oz |
| 15 | lb | kg |
| 16 | kg | lb |
| 17 | °F | °C |
| 18 | °C | °F |
| 19 | gal(US) | l |
| 20 | l | gal(US) |
| 21 | gal(UK) | l |
| 22 | l | gal(UK) |
| 23 | fl oz(US) | ml |
| 24 | ml | fl oz(US) |
| 25 | fl oz(UK) | ml |
| 26 | ml | fl oz(UK) |
| 27 | J | cal |
| 28 | cal | J |
| 29 | hp | kW |
| 30 | kW | hp |
| 31 | ps | kW |
| 32 | kW | ps |
| 33 | kgf/cm2 | Pa |
| 34 | Pa | kgf/cm2 |
| 35 | atm | Pa |
| 36 | Pa | atm |
| 37 | mmHg | Pa |
| 38 | Pa | mmHg |
| 39 | kgfm | J |
| 40 | J | kgfm |
































































